Membrane electrolysis sodium hypochlorite janareta na'ura ce mai dacewa don lalata ruwan sha, kula da ruwa mai tsabta, tsaftar muhalli da rigakafin annoba, da samar da masana'antu, wanda Yantai Jietong Water Treatment Technology Co., Ltd., Cibiyar Nazarin Ruwa ta China da Cibiyar Bincike ta Ruwa, Jami'ar Qingdao, Jami'ar Yantai da sauran cibiyoyin bincike da jami'o'i suka haɓaka. Yana da wani nau'i na inji don samar da high-concentration sodium hypochlorite mafita a wurin, sosai gamsu da bukatar babban taro sodium hypochlorite kayayyakin, da kuma warware harkokin sufuri da kuma ajiya matsaloli. Membrane sodium hypochlorite janareta wanda Yantai Jietong Water Treatment Technology Co., Ltd ya kera shi ne kawai kamfanin fasaha a kasar Sin wanda zai iya samar da babban taro sodium hypochlorite kayayyakin a wurin. Membrane electrolysis brine sodium hypochlorite janareta na iya samar da 4-12% babban taro sodium hypochlorite bayani tare da rufaffiyar madauki na dosing da samar da cikakken sarrafa kansa aiki.
Abubuwan da ke gaba shine Ka'idar Aiki
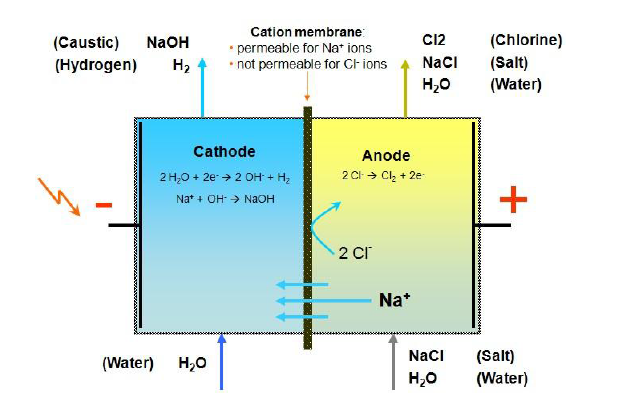
Babban ka'idar amsawar electrolytic na cell electrolysis cell shine canza makamashin lantarki zuwa makamashin sinadarai da electrolyze brine don samar da NaOH, Cl2 da H2 kamar yadda aka nuna a hoton da ke sama. A cikin ɗakin anode na tantanin halitta (a gefen dama na hoton), brine yana ionized cikin Na + da Cl- a cikin tantanin halitta, inda Na + ke ƙaura zuwa ɗakin cathode (gefen hagu na hoton) ta hanyar zaɓaɓɓen membrane ionic a ƙarƙashin aikin cajin. Ƙananan Cl- yana haifar da iskar chlorine a ƙarƙashin anodic electrolysis. H2O ionization a cikin ɗakin cathode ya zama H + da OH-, inda OH- ke toshe shi ta hanyar zaɓaɓɓen membrane cation a cikin ɗakin cathode kuma Na+ daga ɗakin anode an haɗa shi don samar da samfurin NaOH, kuma H + yana haifar da hydrogen a ƙarƙashin electrolysis na cathodic.


Lokacin aikawa: Mayu-31-2024

